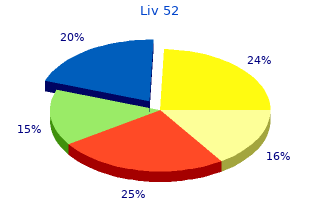
Liv 52
2018, The College of New Jersey, Wilson's review: "Liv 52 200 ml, 120 ml, 100 ml, 60 ml. Discount Liv 52 online in USA.".
Kierdorf H: Continuous versus intermittent treatment: Clinical results 154:268–282 in acute renal failure order 120 ml liv 52 with visa medicine lodge treaty. Bellomo R, Mansfeld D, Rumble S, et al: Acute renal failure in critical arrest care: 2010 American Heart Association Guidelines for Car- illness. Conventional dialysis versus acute continuous hemodiafltra- diopulmonary Resuscitation and Emergency Cardiovascular Care. Nephron 1995; 71:59–64 lin therapy for the management of glycemic control in hospitalized 362. Ann Intern Med 2011; 154:260–267 acute renal failure patients in the intensive care unit. Jacobi J, Bircher N, Krinsley J, et al: Guidelines for the use of an renal replacement therapy for acute renal failure in intensive care insulin infusion for the management of hyperglycemia in critically ill units: Results from a multicenter prospective epidemiological survey. Tonelli M, Manns B, Feller-Kopman D: Acute renal failure in the inten- concentration and short-term mortality in critically ill patients. Anes- sive care unit: A systematic review of the impact of dialytic modality thesiology 2006; 105:244–252 on mortality and renal recovery. J Diabetes Sci Technol 2009; 3:1292–1301 trial comparing intermittent with continuous dialysis in patients with 348. Kanji S, Buffe J, Hutton B, et al: Reliability of point-of-care testing Nephrol Dial Transplant 2005; 20:1630–1637 for glucose measurement in critically ill adults. Vinsonneau C, Camus C, Combes A, et al; Hemodiafe Study Group: 33:2778–2785 Continuous venovenous haemodiafltration versus intermittent hae- 350. John S, Griesbach D, Baumgärtel M, et al: Effects of continuous Trials Group, Cook D, Meade M, Guyatt G, et al: Dalteparin ver- haemofltration vs intermittent haemodialysis on systemic haemody- sus unfractionated heparin in critically ill patients. New Engl J Med namics and splanchnic regional perfusion in septic shock patients: A 2011; 364:1305–1314 prospective, randomized clinical trial. Chest 2007; 131:507–516 parison of the hemodynamic response to intermittent hemodialysis 394. Intensive Care Med 1996; 22:742–746 patients with severe renal insuffciency with the low-molecular-weight 374. Am Surg 1998; 64:1050–1058 vival and recovery of renal function in intensive care patients with 396. A randomized trial comparing 2002; 30:2205–2211 graduated compression stockings alone or graduated compression 376. Mathieu D, Neviere R, Billard V, et al: Effects of bicarbonate therapy vein thrombosis with low molecular-weight heparin in patients under- on hemodynamics and tissue oxygenation in patients with lactic aci- going total hip replacement: A randomized trial. Scott Med J 1981; thrombotic therapy and prevention of thrombosis, 9th ed: Ameri- 26:115–117 can College of Chest Physicians Evidence-Based Clinical Practice 384. Chest 2012; 141(Suppl 2):7S–47S prevention of fatal pulmonary embolism in patients with infectious 403. Lancet 1996; phylaxis of acute upper gastrointestinal bleeding in high risk patients. Prophylaxis in Medical Patients with trointestinal hemorrhage in critically ill patients. Canadian Critical Care Trials Association of Non-University Affliated Intensive Care Specialist Group. Kupfer Y, Anwar J, Seneviratne C, et al: Prophylaxis with subcuta- cal intensive care unit. Am J Med 1984; 76:623–630 neous heparin signifcantly reduces the incidence of deep venous 409. Am J Crit Care Med 1999; mechanically ventilated patients: Integrating evidence and judgment 159(Suppl):A519 using a decision analysis. Crit Care Med and the Australian and New Zealand Intensive Care Society Clinical 2010; 38:2222–2228 Critical Care Medicine www. National Heart, Lung, and Blood Institute Acute Respiratory Distress enteric infection in patients taking acid suppression. N Engl J Med 1998; 338:791–797 intensive insulin therapy in critically ill patients: A randomized con- 415. Lin P, Chang C, Hsu P, et al: The effcacy and safety of proton pump trolled trial. Am J Clin Nutr 2011; 93:569–577 inhibitors vs histamine-2 receptor antagonists for stress ulcer bleed- 436.
Managing wetlands: frameworks for managing Wetlands of International Importance and other wetland sites buy cheap liv 52 60 ml line treatment 5cm ovarian cyst. Chapter 4, Field manual of wildlife diseases: general field procedures and diseases of birds. Revue scientifique et technique (International Office of Epizootics), 21, (1): 159-178. Restrictions on the movements of domestic animals and people, usually imposed by government authorities, can therefore be an effective tool for preventing and controlling disease transmission by reducing contact between infected and susceptible animals. Such measures are particularly useful in wetland sites with substantial human activity, such as human residencies, intensive livestock production, large numbers of visitors or hunters, captive breeding and/or translocation programmes. Movement restrictions to prevent an outbreak Preventative measures may be taken as a response to periods of elevated risk of an outbreak affecting a wetland. In the event of a disease outbreak near to a wetland or at a national level, implementation of animal movement restrictions may be considered a prudent measure. Where a disease outbreak is considered serious, national stock ‘standstills’ may be imposed which restrict all animal movement. It is also important to note that movements of people may also be restricted to and from a wetland. Trade in animals and derived products may also be prohibited locally, nationally or internationally. Movement restrictions to control an outbreak Rapid notification of the presence of disease by wetland managers is vital for the timely mobilisation of control activities. The overall cost of a disease management strategy may be reduced if disease is prevented or controlled at an early stage during the outbreak, and economic impacts related to restricted animal trade will be minimised. If a notifiable disease is confirmed in domestic animals and/or wildlife at a wetland site, there are likely to be automatic movement restrictions placed on people and animals by government authorities to reduce the risk of further spread. During such an outbreak stock must not be moved within or external to the site until restrictions are lifted: contravention of statutory movement restrictions can result in criminal prosecution. The site contingency plan should be implemented and personnel guided through the process in the event of a disease outbreak [►Section 3. Controls may be implemented whereby movements of susceptible species are only permitted under strict, designated conditions, when it is deemed safe. When such activities are allowed to resume, they should be subject to surveillance and rigidly enforced codes of practice. If area restrictions have been imposed on a site, visits to other wetland sites or areas with livestock should only take place if they are essential and should be subject to strict biosecurity measures [►Section 3. Until a disease outbreak is brought under control, rights of way through the infected area should be closed and non-essential visits to infected sites should be suspended. Infected or potentially infected sites, animals and their products, personnel, potentially contaminated animal products and other materials may be placed under quarantine. Appropriate health restrictions can be placed on the movement of susceptible animals into, or out of, the quarantine area until the infection is considered to have been removed. This may be supported by disinfection and decontamination of personnel, vehicles, equipment and other materials leaving and entering the quarantine area [►Section 3. Quarantine guidelines vary depending on the case and factors involved (disease, terrain, local human and animal populations) but will generally cover at least a 3-5 km radius from the initial case. Movement restrictions are often imposed over a wider area around the quarantined or infected site as part of a zoning strategy which seeks to identify disease infected, disease-free and buffer zone areas [►Section 3. The coverage of the outbreak area and surrounding areas of risk can be determined from surveillance activities and relies on an understanding of the epidemiology of the disease and host ecology [►Section 3. Animal movement within identified zones is not permitted unless appropriate permits have been issued by the local authorities. Trade in certain animals and their products may be permitted under particular circumstances from disease-free zones but only where this has been authorised. Controlled area restrictions may apply whereby the movement of animals outside the protection and surveillance zones is controlled. Imposed movement restrictions and other disease control activities should be communicated promptly and clearly to relevant stakeholders and local communities by local authorities [►Section 3. An integrated disease management strategy, which includes a range of disease control activities such as movement restrictions, zoning, surveillance and vaccination, is often most effective. A disease management strategy for the site should incorporate how best to respond to and cope with movement restrictions. Consideration should be given to voluntary implementation at times of increased risk (e.
Other studies have found a similar beneft of improving surveillance for infectious diseases via automatic notifcation with electronic medical records (Allen and Ferson purchase 60 ml liv 52 with mastercard symptoms kidney infection, 2000; Hopkins, 2005). Standardized Laboratory Reporting It is essential that laboratory data be standardized and that health departments have automated access to them. Automated electronic laboratory reporting improves the completeness and timeliness of disease surveillance (Effer et al. Local health departments have to investigate all positive hepatitis B tests in women of childbearing age, and this creates a substantial workload. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C http://www. A review of the literature evaluating the timeliness of reporting of infectious diseases found that reporting lag and the variability among states limit the usefulness of data. The review called for a more standardized approach in evaluating and describing surveillance- system timeliness (Jajosky and Groseclose, 2004). Although it did not look specifcally at hepatitis B or hepatitis C, its conclusions are relevant to the present report. Electronic Medical Records The reporting of relevant infectious-disease test results should be a component of electronic medical-record systems. Case Investigation and Followup Standards for case investigation and followup should be developed and implemented to ensure that newly diagnosed patients receive ad- equate information and referrals. Identifcation of infected people by health departments should be the frst step in getting them into appropriate care. As discussed earlier in this chapter, there are important concerns about underreporting, particularly of the incidence of Copyright © National Academy of Sciences. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C http://www. Until the quality of the data collected has improved, reports should clearly indicate the limitations of the data. Targeted Surveillance Once core hepatitis B and hepatitis C surveillance activities are well established, supplemental or pilot projects should be tested. The Centers for Disease Control and Preven- tion should support and conduct targeted active surveillance, including serologic testing, to monitor incidence and prevalence of hepatitis B virus and hepatitis C virus infections in populations not fully captured by core surveillance. Serosurveillance projects will provide data for improved estimation of the scope of the problem in underrepresented populations such as certain racial and ethnic groups, and at-risk populations, including institutional- ized, homeless, immigrant, and refugee populations. Enhanced surveillance projects should be structured to obtain information in both rural and urban regions of the United States. Conducting serosurveillance or screening among at-risk populations in correctional facilities may provide opportunities to Copyright © National Academy of Sciences. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C http://www. Other enhanced surveillance projects should include • Determining the level of care that patients receive after diagno- sis, including medical and social-service referrals and treatment (Fleming et al. Notifcation of infectious diseases by general practitioners: A quantitative and qualitative study. Guidelines for laboratory testing and resultGuidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C http://www. Prevention of perinatal hepatitis B through enhanced case management—Connecticut, 1994-95, and the United States, 1994. Guidelines for national human immunodefciency virus case surveillance, includ- ing monitoring for human immunodefciency virus infection and acquired immunodef- ciency syndrome. National hepatitis C prevention strategy: A comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences. Updated guidelines for evaluating public health surveillance systems: Recom- mendations from the guidelines working group. Hepatitis C virus transmission from an antibody-negative organ and tissue donor—United States, 2000-2002. Prevention and control of infections with hepatitis viruses in correctional set- tings. Transmission of hepatitis B and C viruses in outpatient settings—New York, Oklahoma, and Nebraska, 2000-2002. Transmission of hepatitis B virus among persons undergoing blood glucose mon- itoring in long-term-care facilities—Mississippi, North Carolina, and Los Angeles county, California, 2003-2004.