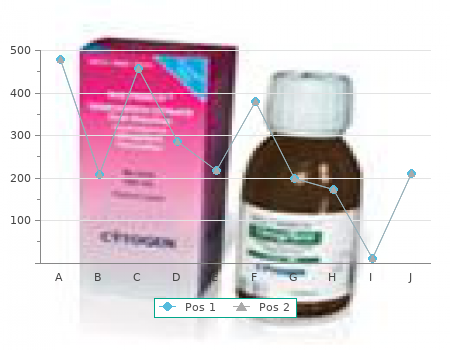
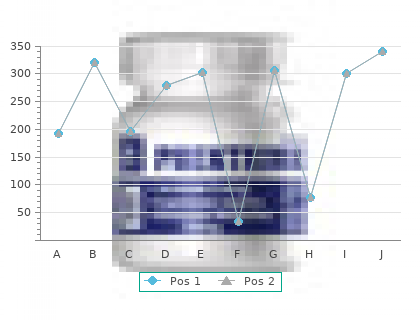
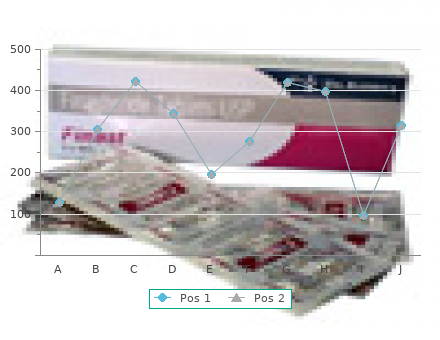
Tinidazole
By X. Angar. Southern Wesleyan University. 2018.
Serotonin glucuronidation was doubled in microsomes from persons with moderate-to-heavy alcohol use (54) order tinidazole 500mg mastercard antimicrobial fabric manufacturers. Consequently, the mechanism of induction by oral contraceptives, phenytoin, and rifampin is unclear and may involve multiple enzymes. Propofol clearance is greater than liver blood flow, also suggesting that extrahepatic metabolism is important for this compound. A number of pharmacodynamic interactions have been reported between propofol and benzodiazepines or opoids such as fentanyl and alfentanil (68–70). Phar- macokinetic interaction studies in humans with fentanyl or alfentanil revealed a modest decrease in propofol clearance (20–50%). The 7-O-glucuronide is the predominant conjugate formed in vivo and is the major excretory metabolite of mycophenolate (90% of the dose in human urine). The two regulatory region mutations are more common appearing in >15% of Caucasians and may result in increased protein expression. In a population of 95 kidney transplant recipients, (83) 16/95 carried only the –275 T>A mutation, 12/95 had only the –2152 C>T mutation, and 11/ 95 carried both mutations, although Innocenti et al. Allele frequencies were 60% in Japanese (n ¼ 87), 39% in Caucasians (n ¼ 50), and 44% in African Americans (n ¼ 50). Innocenti found similar frequencies [53% in Asians (n ¼ 200) and 39% in Caucasians (n ¼ 254)] (84). In vitro, the enzyme was shown to catalyze conjugation at both the phenolic hydroxyl at the 7- position and the carboxylic acid moiety to form an acyl glucuronide. These data suggest that the Caco-2 cell system may not be the optimal model to predict small intestinal glucur- onidation. The very low bioavailability of raloxifene in humans (2%) is therefore 100 Remmel et al. The Vmax values for the M59I variant were about half of wild type for 17b-estradiol glucuronidation with a similar Km value (93,94). This enzyme had activity toward several steroidal substrates, including estriol and androsterone, with low activity for the bile acid and hyodeoxycholic acid. Morphine glucuronidation has been well studied; however, relatively few clinical drug-drug interaction with morphine have been reported. However, morphine-6-glucuronide has poor ability to cross the blood-brain barrier, with a permeability coefficient in rats that 1/57 that of morphine. Morphine-6-glucur- onide has potency similar to the analgesic effects of morphine when administered to rats on a mg/kg basis. In humans, both morphine-3-glucuronide (lacking analgesic activity) and morphine-6- glucuronide are present in higher concentrations in plasma than morphine at steady state. The data did not fit to a simple hyperbolic fit expected of a competitive inhibitor of single enzyme. Drug interaction studies with lorazepam and clofibric acid in humans have been reported and are summarized in Table 5. The location of this amino acid change is near the junction of the variable and constant regions (99). There was no functional polymorphism observed for seven common genotypes and the three main haplotypes with regard to the morphine-6- glucuronide/morphine ratio. A similar study on the effect of polymorphisms on morphine kinetics was done in the United States by Sawyer et al. In this study, morphine-6-glucuronide and morphine-3-glucuronide concentrations were significantly lower in C/C patients (105). Median rates of glucuronidation were 65 pmol/min/mg protein in male samples (25–75% range of 49–112, n ¼ 38) versus 39 in females (25–75% range of 30–72, n ¼ 16), p ¼ 0. Probenecid inhibits the active tubular secretion of a number of organic anions, including uric acid and glucuronides of several different drugs. In addition to the expected effect of a decreased rate of glucuronide excretion, these studies have also revealed that the clearance of the parent aglycone is also decreased. In several cases, it has been demon- strated that probenecid affects both the nonrenal and renal clearance of the parent aglycones, suggesting that there are multiple mechanisms for the probenecid effect. Similarly, both the phenolic and acyl glucuronide formation clearance of diflunisal was reduced by approximately 50% (114). An alternate mechanism involving hydrolysis of the glucuronide back to the parent aglycone has also been proposed. The reversible metabolism (futile cycle) hypothesis has been well studied with clofibric acid in a uranyl nitrate–induced renal failure model in rabbits (123).
This conclusion is based on over 500 cases of dental work quality tinidazole 1000 mg antimicrobial susceptibility, all free of antibiotics and infection. I recommend hot packing be- cause I consider swelling less important than infection or pain, especially if you are not on an antibiotic. Antibiotics are too unreliable for cancer patients, with one exception, heart disease. Typically, how- ever, you can look forward to your jaw healing stronger than ever, a boost of health, and no antibiotics or side effects! Dental Aftercare, Heal The Jaw To heal your jaw bone after dental work you need extra cal- cium, magnesium and vitamin D. Because most supplements are highly processed, and therefore contain trace amounts of solvents and heavy metals, it is wiser to use the food nature in- tended for growing bones. Goats’ milk or cows’ milk has the extra calcium (one gram per quart) you now need. Many harmful bacteria ride along from the dairy barn, through the milk tanks and into your milk container. Salmonellas and Shi- gellas are two very harmful bacteria always found in every milk sample I test. Besides bacteria, one can find eggs of parasites, such as tapeworms and flukes in milk. And since cheese, yogurt, ice cream and butter are made from milk, they too are contami- nated, in spite of pasteurization. Of course, you could test your dairy products with a Syncrometer to try to find a good one. The salt raises the boiling tem- perature just enough to kill Rhizobium leguminosarum, too, which is extra hardy. Milk also has traces of malonic acid, a strong metabolic in- hibitor, and boiling does not detoxify it. This could curdle some milk, so an equal amount of baking soda may be added first. Persons with anemia should buy raw milk in order to obtain the factor, lactoferrin. Raw goat milk is best because it has some of the same factors as shark cartilage in addition to lac- toferrin. So, to upgrade your dental health, begin by increasing your calcium intake with milk and fish. Get the extra magnesium you need from leafy green vege- tables plus a supplement (magnesium oxide, 300 mg daily). There are three hazards with eating greens: pesticides, As- caris eggs and sprays. If you are not sure whether pesticides have been used, then only buy Swiss chard, cabbage, collards (large leafed greens) that can be easily washed. One drop of Lugol’s iodine in one quart of water is strong enough to kill on contact. If you see spray nozzles in the produce section, you must detoxify any benzene that may be present with ozone. To get rid of the phenol, soak the greens for five min- utes in a bowl of water with a pinch of baking soda added. Dental Aftercare Summary You are hot-packing, hot-swishing, and water picking many times a day. You are flossing and brushing your remaining teeth with white iodine or colloidal silver. You now have the extra calcium, magnesium and vitamin D you need to heal your jaw bone. If bleeding and pain do not stop by the third day, you will return to your doctor before your stitch-removal appointment day. The toxins I am referring to I call the “M-Family” and consist of malonic acid (also called malonate), methyl malonic acid, maleic acid, maleic anhydride, and D-malic acid. Malonic acid is widely used in organic chemical manu- 30 facturing and most dental plastics test positive to it, too, making it a common pollutant. Maleic acid is a component of 31 some bonding agents (meant for bonding the plastic to the tooth).
Water order 500 mg tinidazole otc infection lymph node, for example, freezes at 08C, and it does so regardless of the amount of water being frozen, so ice cubes and icebergs freeze at the same temperature. When Km is found to increase with Vmax, it is more than likely that the metabolism of the substrate was not 320 Ogilvie et al. Therefore, sample-to-sample variation in Km values, particularly when such variation coincides with the variation in Vmax values, is usually an experimental artifact. However, it should be noted that great care was taken to measure initial rates of coumarin 7-hydroxylation. The percentage of substrate converted to 7-hydroxycoumarin ranged from less than 1% to about 15%. It was speculated that reports of higher Km values for the 7-hydroxylation of coumarin by human liver microsomes, such as a Km of 10 mM reported by Yamazaki et al. The experiment designed to evaluate the effect of incubation time and protein concentration on the formation of metabolites (Step 2) provides the preliminary data necessary to select a range of substrate concentrations and experimental conditions to determine Km and Vmax for the metabolism of the drug candidate by human liver microsomes. A crude estimation of Km can be obtained from the three substrate concentrations used in Step 2, provided rates of metabolite formation represents initial reaction velocities. Km and Vmax should be measured with a 100-fold range of substrate concentrations, one that ranges from one-tenth Km to ten times Km. However, this range of substrate concen- trations may have to be expanded if metabolite formation is catalyzed by two kinetically distinct enzymes (one with low and one with high Km). The kinetic constants (Km and Vmax) for a given reaction are usually determined with a pool of human liver microsomes as follows. Typically, the pool of human liver microsomes (single protein concentration) are incubated in triplicate for a specified time period with a drug candidate (e. For all substrate concentrations, the rate of reaction is measured under initial rate conditions; that is, the product formation is directly proportional to protein concentration and incubation time and the percentage metabolism of the substrate does not exceed 10%. Initial rate conditions may be achieved by varying the incubation time or the protein concentration, if necessary. Note that, in some cases, poor substrate solubility may prevent metabolite formation being measured at high substrate concentrations (especially at 10Km). Alternatively, low analytical sensitivity may impede the detection of metabolite formed at substrate concentrations well below Km (especially one-tenth Km). Enzyme kinetic constants are calculated by nonlinear regression analysis with computer software, such as GraFit (Erithacus Software Limited, In Vitro Study of Drug-Metabolizing Enzymes 321 Figure 22 Examples of enzyme kinetic plots used for determination of Km and Vmax for a normal and an allosteric enzyme: Direct plot [(substrate) vs. K0 is a con- m max stant that incorporates the interaction with the two (or more) binding sites but that is not equal to the substrate concentration that results in half-maximal velocity, and the symbol “n” (the Hill coefficient) theoretically refers to the number of binding sites. It should be noted that, at times, nonlinear regression lines represent the data points on an Eadie-Hofstee plots very poorly because the data reflect the contribution from two kinetically distinct enzymes whereas the computer software attempts to fit all data to an equation appropriate for a single enzyme. A relatively high standard error associated with the estimate of Km suggests that the nonlinear regression did not fit the data very well, and it is possible that a two enzyme model or perhaps an atypical enzyme kinetics model needs to be selected. When Km values are estimated by extrapolating data beyond the concentration range 322 Ogilvie et al. If the standard error associated with the Km value is large (>25%) and/or if the Km value falls outside the range of substrate concentrations studied, it is prudent to repeat this exper- iment with a new range of substrate concentrations that bracket the estimated Km value. When the Eadie-Hofstee plot suggests the involvement of two kinetically distinct enzymes in the formation of a particular metabolite, the data should be fitted to a dual-enzyme model according to the following equation: Vmax1 Á ½S Vmax2 Á ½S vtotal ¼ v1 þ v2 ¼ þ ð8Þ Km1 þ ½S Km2 þ ½S where vtotal is the overall rate of metabolite formation at substrate [S], Vmax1 and Vmax2 are the maximal velocities of the reaction, and Km1 and Km2 are the Michaelis-Menten constants for enzyme 1 and enzyme 2, respectively. For simplicity, the following discussion assumes that enzyme 1 is the high-affinity (low-Km) enzyme and that enzyme 2 is the low-affinity (high-Km) enzyme. It further assumes that Km1 and Km2 differ by at least an order of magnitude and that the range of substrate concentrations extended well below Km1 and up to or above Km2. Under such conditions, enzyme 2, the high-Km enzyme, contributes negligibly to vtotal at low substrate concentrations, and the range of substrate concentrations where this is largely true can be identified by visual inspection of the Eadie-Hofstee plot; (Fig. These “enzyme 1” data are plotted on an Eadie-Hofstee plot to obtain Km1 and Vmax1. Subsequently, v2 (which equals vtotal À v1) is calculated, and the data are plotted on an Eadie-Hofstee plot to obtain Km2 and Vmax2. When Km1 and Km2 differ by less than an order of magnitude, or when the range of substrate concentrations does not bracket both Km1 and Km2, it may not be possible to determine the kinetic constants of the individual enzymes. Two enzymes with similar Km values toward the same substrate have frequently been observed, and these will result in an Eadie-Hofstee plot consistent with single-enzyme kinetics.
Practitioners should check their individual organisation’s policies in order to determine when the use of a heparin flush is appropriate tinidazole 1000mg discount bacteria pictures. Central versus peripheral access Before a medicine can be administered intravenously, a suitable intravenous catheter must be inserted into the patient. Choice of line is often dictated by the clinical condition of the patient; for example, a critically ill patient is more likely to require central access than a patient who only requires a few days’ intravenous therapy. Unlike peripheral catheters, central lines are generally thought to last longer, are more consis- tently accessible regardless of the patient’s condition and can accept more irritant solutions, e. Central catheters also carry the advantage of allowing a rapid rate of infusion of a drug and can be used to monitor central venous pressures as well as aid the passing of pulmonary artery catheters. However, insertion of central intravenous catheters requires greater skill and the risks associated with them are greater. Central and peripheral catheters are often referred to in the vernacular as central lines and peripheral lines. The term glucose (abbreviated to Gluc in this book) is used throughout the monographs to define the injection and infusion fluid of that substance. The term dextrose is used by some authorities and on occasion this been known to lead to confusion. Glucose is a three-dimensional molecule that exists in two symmetrical forms (mirror images of each other) and the dextro-andlaevo- prefixes are used to categorise the forms that can rotate plane- polarised light clockwise (to the right) or anticlockwise (to the left) respectively. The word dextrose is a contraction of dextrorotatory glucose or d-glucose, which is the biologically active form of glucose, as opposed to laevorotatory glucose or l-glucose (which is not biologically active). It is however structurally different from glucose and is not the laevorotatory form of glucose. Osmolarity Osmolarity is stated in some monographs only for those drugs that have a high osmolarity. The significance of this is that at a solution of high osmolarity can cause serious harm to small blood vessels and these drugs would not normally be appropriate for peripheral administration. It is worth explaining what the term means and how it differs from the similar term osmolality. Osmolarity is a measure of solute concentration in terms of osmoles per litre of solution (Osmol/L), whereas osmolality is a measure of the number of osmoles of solute per kg of solvent (Osmol/kg). Calculated figures for both are very similar and the terms are used interchangeably in practice with little consequence. An osmole (Osm or Osmol) is a unit of measure that describes the number of moles of a chemical compound that contribute to a solution’s osmotic pressure, that is, the pressure that must be applied to a solution to prevent the inward flow of water across a semi-permeable membrane. A ppendix 2 G ood m anagem ent principles It is unacceptable to prepare medicines for administration by others. The practitioner administering the drug must be involved in the preparation and checking process. The following are exemptions from this principle: * Injections and infusions prepared by the Pharmacy department. Parenteral drugs must not be prepared in advance of their immediate use (except when prepared by the Pharmacy). Parenteral medicines do not routinely need to be double-checked by another practitioner; however, each practitioner should check their own organisation’s policy on this matter before administering a medicine. It is good practice to request a double check on administration if: * The infusion involves the addition or mixing of drugs, e. Local circumstances may make involvement of a second practitioner desirable in order to min- imise the potential for error, e. Each practitioner is responsible for maintaining his or her own knowledge on all drugs. Information on such products can be found in the monographs of this book, in the package insert and/or via the Pharmacy department. Accountability Parenteral therapy is a common and important part of the care received by many patients. In order to protect patients and provide staff withthemeanstodeliversafeandeffectivetreatment,thepractitionershouldalwaysfollowguidance issued by their own organisation. In order to provide comprehensive patient care all nurses/midwives are expected to achieve competency at the earliest opportunity following appointment and may be called on to demonstrate their competency at any time. Although nurses/midwives can decline to perform duties in which they do not feel competent, they are obliged to adapt to new methods and techniques of adminis- tering medications and must work at a level commensurate with the grading of their role.