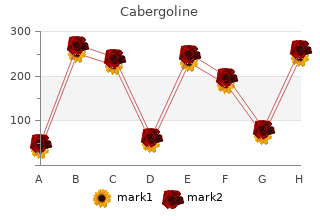
Cabergoline
By G. Campa. John Jay College of Criminal Justice.
This enzyme converts lanosterol to ergosterol generic 0.5 mg cabergoline visa womens health issues, and is required in fungal cell-wall synthesis. Allylamines (naftifine, terbinalfine, butenafine, amorolfine) inhibit the enzyme squalene epoxidase, another enzyme required for ergosterol synthesis. Others: Griseofulvin binds to polymerized microtubules and inhibits fungal mitosis. From the medical point of view, antifungal drugs are con- sidered dermatophytic, mucocutaneous, and systemic. Amphotericin B: Amphotericin B, [1R(1R∗,3S∗,5R∗,6R∗,9R∗,11R∗,15S∗,16R∗,17R∗, 18S∗,19E∗,21E∗,23∗,25E∗,27E∗,29E∗,31E∗,33R∗,35S∗,36R∗,37S∗)]-33-[3-amino-3,6- dideoxy-β-D-mannopyranosyl)-oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl- 13-oxo-14,39-dioxabicyclo [33. The antifungal activity of amphotericin B is exhibited because it binds with sterols, in particular with ergosterol in the cellular membrane of sensitive fungi. This reaction makes pores in the membrane and increases the perme- ability of the membrane to small molecules, thus reducing the function of the membrane as an osmotic barrier and making the cells more sensitive to being destroyed. However, this compound is not highly selective and reacts with host mammalian cells. Despite the many side effects, amphotericin B remains the primary drug for treating severe, acute systemic fungal infections. It is used for generalized fungal infections, such as can- didomycosis, aspergillosis, histoplasmosis, cryptococcosis, coccidioidomycosis, blasto- mycosis, and pulmonary mycoses. The mechanism of antifungal activity is similar to the mechanism of action of ampho- tericin B. It is used for preventing and treating candida infections of the skin and mucous membranes. In terms of preventative action, it is used for preventing the development of candidomycosis during prolonged treatment with penicillin drugs and antibiotics of other group, especially during oral use of tetracycline antibiotics, levomecytin, and others. Synonyms of this drug are biofanal, moronal, nicporil, fazigin, candex, and others. Natamycin: Natamycin, a mixture of stereoisomeric 22-[(3-amino-3,6-dideoxy-β-D- mannopyranosyl)oxy]-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxatricy- clo[22. It exhibits especially pronounced activity against a few strains of Fusarium and Cefalosporium. Natamycin is a drug for treating superficial fun- gal infections, and it is used only for ophthalmologic purposes. They are ketoconazole, miconazole, clotrimazole, econazole, butoconazole, and others. Antifungal Drugs The antifungal activity of imidazole derivatives is currently explained by their ability to selectively raise the permeability of the membrane of fungal cells by interfering with the biosynthesis of sterins, in particular ergosterin, by inhibiting its synthesis and by changing the lipid content of the membrane. However, unlike amphotericin B, benzimidazole deriv- atives are active only against growing cells. This drug does not affect host cells because mammals use exogenic sterols for their vital functions. Acylating the hydroxyl group of this compound with benzoyl chloride, and then alkylating the resulting compound with imidazole gives the derivative (35. Next, alkaline hydrolysis removes the benzoyl group, and a reaction with methanesulfonyl chloride gives a mesylate (35. Finally, alkylating the resulting 1-acetyl-4-(4-hydroxyphenyl)piperazine gives ketoconazole (35. It possesses fungicidal and fungistatic activity with respect to dermatophytes, yeast fungus, dimorphous fungi, and eumycetes. It is effective for chronic diseases, treating fungal infections of the gas- trointestinal tract, sex organs, skin, hair, and nails. It is used in combination with shampoo for treating and preventing mycelial fungi, seborrheic dermatitis, and dandruff. Reducing the carbonyl group in this molecule with sodium borohydride gives 1-(2,4-dichlorophenyl)-3-(1-imidazolyl)-ethanol (35. It differs in the presence of a single chlorine atom in the benzyl part of the molecule, and it is synthesized in the same manner, except that it uses 4-chlorobenzylchloride in the last stage instead of 2,4-dichlorobenzylbromide [22–24]. It differs in the replacement of the etheral oxygen bridge (which connects the 4-chlorobenzyl part of the molecule with phenethylimidazole) for a thioether bond.
In other words generic 0.5 mg cabergoline with mastercard women's health clinic vero beach, to create gas bubble of radius R in a liquid with surface tension T, the pressure of the gas injected into the liquid must be greater than the pressure of the surrounding liquid by P as given in Eq. As will be shown in the following sections, the effects of surface tension are evident in many areas relevant to the life sciences. These spaces act as capillaries and in part govern the motion of water through the soil. When water enters soil, it penetrates the spaces between the small particles and adheres to them. If the water did not adhere to the particles, it would run rapidly through the soil until it reached solid rock. Because of adhesion and the resulting capillary action, a significant fraction of the water that enters the soil is retained by it. For a plant to withdraw this water, the roots must apply a negative pressure, or suction, to the moist soil. For example, if the effective capillary radius of the soil is 10−3 cm, the pressure required to withdraw the water is 1. Because capillary action is inversely proportional to the diameter of the capillary, finely grained soil will hold water more tightly than soil of similar material with larger grains (see Fig. When all the pores of the soil are filled with water, the surface mois- ture tension is at its lowest value. In other words, under these conditions the required suction pressure produced by the plant roots to withdraw the water from the soil is the lowest. As the soil loses moisture, the remaining water tends to be bound into the narrower capillaries. In addition, as the moisture content decreases, sec- tions of water become isolated and tend to form droplets. If, for example, the radius of a droplet decreases to 10−5 cm, the pressure required to draw the water out of the droplet is about 14. Capillary action also depends on the strength of adhesion, which in turn depends on the material composition of the capillary surface. There is a limit to the pressure that roots can produce in order to withdraw water from the soil. A plant may thrive in loam and yet wilt in a clayey soil with twice the moisture content. Many of these insects are adapted to utilize the surface tension of water for locomotion. The surface tension of water makes it possible for some insects to stand on water and remain dry. As is shown in Exercise 7-11, a 70 kg person would have to stand on a platform about 10 km in perimeter to be supported solely by surface tension. Further, examination with an electron microscope reveals that the myofibril is composed of two types of threads, one made of myosin, which is about 160 A(˚ 1A˚ 10−8 cm) in diameter, and the other made of actin, which has a diameter of about 50 A. The threads are aligned in a regular pattern with spaces between threads so that the threads can slide past one another, as shown in Fig. The calcium ions in turn produce conformational changes that result in the sliding of the threads through each other, shortening the myosin-actin structure. Clearly, a force must act along the myosin-actin threads to produce such a contracting motion. It has been suggested by Gamow and Ycas [7-5] that this force may be due to surface tension, which is present not only in liquids but also in jellylike materials such as tissue cells. Here the movement is due to the attraction between the surfaces of the two types of thread. Let us now estimate the force per square centimeter of muscle tissue that could be generated by the surface tension proposed in this model. If the average diameter of the threads is D, the number of threads N per square centimeter of muscle is approximately 1 N (7. There- fore, the maximum contracting force that can be produced by surface tension per square centimeter of muscle area is 6 2 Fm T × 4 × 10 dyn/cm A surface tension of 1. Because this is well below surface tensions commonly encountered, we can conclude that surface tension could be the source of muscle contraction. The actual processes in muscle contraction are much more complex and cannot be reduced to a simple surface tension model (see [7-7 and 7-9]). As the word implies, the hydrophilic end is strongly attracted to water while the hydrophobic has very little attraction to water but is attracted and is readily soluble in oily liquids.
Due diligence has been taken by the publishers purchase cabergoline 0.25 mg line pregnancy labor symptoms, editors, and authors of this book to ensure the accuracy of the information published and to describe generally accepted practices. The contributors herein have carefully checked to ensure that the drug selections and dosages set forth in this text are accurate in accord with the standards accepted at the time of publication. Notwithstanding, as new research, changes in government regulations, and knowledge from clinical expe- rience relating to drug therapy and drug reactions constantly occurs, the reader is advised to check the product information provided by the manufacturer of each drug for any change in dosages or for additional warnings and contraindications. This is of utmost importance when the recommended drug herein is a new or infrequently used drug. It is the respon- sibility of the health care provider to ascertain the Food and Drug Administration status of each drug or device used in their clinical practice. The publisher, editors, and authors are not responsible for errors or omissions or for any conse- quences from the application of the information presented in this book and make no warranty, express or implied, with respect to the contents in this publication. For additional copies, pricing for bulk purchases, and/or information about other Humana titles, contact Humana at the above address or at any of the following numbers: Tel. Photocopy Authorization Policy: Authorization to photocopy items for internal or personal use, or the internal or personal use of specific clients, is granted by Humana Press Inc. The fee code for users of the Transactional Reporting Service is: [1-58829-211-8/04 $25. Use of multiple drugs (8–12 on average in hospitalized patients) is common in a number of therapeutic regimens. In addition to multiple drug therapy, a patient may have access to several prescribers, and may have predisposing illnesses or age as risk factors for interactions. Drug interactions may occur between prescription drugs, but also between food and drug, and chemical and drug. Whereas some may be adverse, interactions may also be sought to decrease side effects or to improve therapeutic efficacy. Pharmacokinetic mechanisms of interaction include alterations of absorption, distribution, biotransformation, or elimination. Absorption can be altered when drugs that alter pH or motility are co-administered, as seen with certain antiulcer or antidiarrheal medications, or when drugs are chelators or adsorbents (tetracyclines and divalent cations, cholestyramine, and anionic drugs). Distribution variations can result from competition for protein binding (sulfa drugs and bilirubin binding to albumin) or displacement from tissue-binding sites (digitalis and calcium channel blockers or quinidine). Induction of gene expression (slow), activation or inhibition (much quicker) of liver and extrahepatic enzymes such as P450, and conjugating enzymes have long found a place of choice in the literature describing the potential for adverse drug interactions resulting from altered metabolism. For example, induction is well described with the major anticonvulsant medications phenytoin, carbamazepine, and barbiturates, whereas inhibition can occur with antimicrobials from the quinolone, the macrolide, and the azole families. Finally, excretion can also be modified by drugs that change urinary pH, as carbonic anhydrase inhibitors do, or change secretion and reabsorption pathways, as probenecid does. Pharmacokinetic interactions in general result in an altered concentration of active drug or metabolite in the body, modifying the expected therapeutic response. A second form of interaction has received little attention because of its modeling complexity and perhaps the poor understanding of basic physiological, biochemical, and anatomical substrates for drug action. Pharmacodynamic interactions involve additive (1 + 1 = 2), potentiating (0 + 1 = 2), synergistic (1 + 1 = 3), or antagonistic (1 + 1 = 0) v vi Preface effects at the level of receptors. Large families of receptors to drugs involve signal transduction pathways and changes in intracellular second messenger concentrations (autonomic nervous system drugs and α, β, muscarinic receptors, for example). Handbook of Drug Interactions: A Clinical and Forensic Guide addresses both types of drug interactions, emphasizing explanations when possible, and careful review of the general pharmacology. The result, we hope, will prove useful to health and forensic professionals as well as medical, pharmacy, nursing and graduate students alike. Brunette Chapter 16: Psychotropic Medications and Crime: The Seasoning of the Prozac Defense..................................... These views are not necessarily the views of the organizations with whom the authors are employed (or otherwise associated), nor the views of the authors of other chapters. Introduction The purpose of this chapter is to examine the drug interactions that occur with ben- zodiazepines and discuss the relevance of these interactions to the field of medicine in general with an emphasis on forensic toxicology. Because of the diverse nature of the benzodiazepines, some time has been taken to introduce this class of drugs. This introductory material has drawn upon some basic reference material and reviews (1–8), and is not otherwise referenced, except for specific points that did not come from these references.
Several case reports of possible associa- tion of this agent with unilateral renal agenesis in the human have been published (Shotton and Monie discount cabergoline 0.5mg without prescription women's health clinic jeddah, 1963; Steege and Caldwell, 1980), but no causal inference can be made. One of five fetuses exposed in the first trimester had a congenital anomaly (Doll et al. Central nervous system anomalies, postcranial skeleton, and palatal closure were increased in frequency among rodents whose mothers were given large doses of chlorambucil during pregnancy (Chaube and Murphy, 1968; Mirkes and Greenaway, 1982; Monie, 1961). Ifosfamide Ifosfamide (Ifex) is a chemotherapeutic agent, chemically related to nitrogen mustards and a synthetic analog of cyclophosphamide, which requires metabolic activation by microsomal liver enzymes to produce biologically active metabolites. Ifosfamide is particularly toxic to the urinary epithelium and must be given with mesna (Mesnex, Uromitexan). It is also used to treat acute leukemias and lung, pancreas, breast, cervix and endometrium cancers, as well as Ewing’s sarcomas, lymphomas, osteosarcoma, soft tissue sarcoma, and ovarian cancer. No epidemiologic studies have been published of congenital anomalies in fetuses whose mothers used this agent during pregnancy. To date there are two case reports of fetuses exposed in utero to ifosfamide-containing com- bination chemotherapy, of which one developed oligohydramnios (Barrenetexa et al. The manufacturer reports that embryotoxic and terato- genic effects have been observed in mice, rats, and rabbits. Two case reports of congenital anomalies after first-trimester combination chemotherapy (Garrett, 1974; Mennuti et al. Chemotherapy with mechlorethamine and other drugs that were discontinued prior to 132 Antineoplastic drugs during pregnancy conception did not increase the frequency of congenital anomalies among more than 40 infants above the 3. No birth defects were reported among the children of 12 women treated with mechlorethamine and other antineoplastic agents during pregnancy in one series (Aviles et al. Increased frequencies of congenital anomalies were found among the offspring of pregnant rodents that were given mechlorethamine in doses several times those normally used in humans (Beck et al. Somatic chromosome breaks have been observed among embryos of pregnant animals who received this agent during gestation (Soukup et al. The relevance of this finding to human reproduction is unknown because gonadal cell lines were not ana- lyzed. It is also used for treatment of chronic myelocytic leukemia, melanoma, osteosarcoma, soft tissue sarcoma, and thyroid cancers. The manufacturer reports that oral melphalan is teratogenic and embryolethal in animals. Although there are no stud- ies of the use of this drug during pregnancy in humans, its strong mutagenic and cyto- toxic actions suggest that it is a likely human teratogen and should be avoided during pregnancy. As with most antineoplas- tics, no epidemiological studies have been published of pregnancy outcome after the use of this agent during human pregnancy. None of four fetuses exposed in the first trimester developed malformations (Doll et al. One case was reported of fetal growth retardation associated with use of the drug in the latter half of pregnancy (Stevens and Fisher, 1965). An increased frequency of congenital anomalies and growth stunting was reported among the offspring of pregnant rodents that received this agent during preg- nancy (Korogodina and Kaurov, 1984; Murphy et al. A patient received carmustine throughout pregnancy and delivered a normal neonate (Schapira and Chudley, 1984). Carmustine must be suspected of being teratogenic because of its Alkylating agents 133 biochemical action (an alkylating agent). Rodents exposed to carmustine at several times the usual human dose during embryogenesis had increased frequency of birth defects (Wong and Wells, 1989). Otherwise, little information is published about the use of this agent during pregnancy in humans or animals. Antimetabolites Antimetabolites can be divided into three groups: folate antagonists, purine antagonists, and pyrimidine antagonists (Box 7. This antineoplastic agent was previously used as an abortifa- cient, but is no longer widely used as an antineoplastic or abortifacient. This finding is relevant to other folate antagonist antineoplastics that are commonly used. The precise risk of congenital anomalies following maternal exposure to this agent is unknown but is likely high (Warkany, 1978). Malformations of the skull, face, eye, and abdominal wall were described among rodents born to mothers that were administered large doses of the aminopterin during pregnancy (Baranov, 1966; Puchkov, 1967). Even at doses lower than those used in humans, malformations were observed in rabbits (Goeringer and DeSesso, 1990).