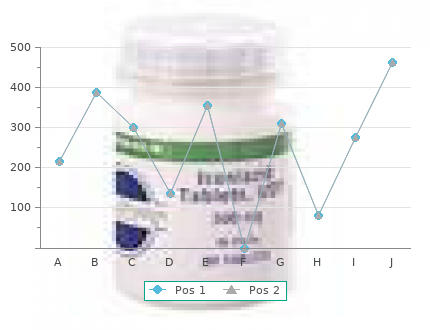
Haldol
By K. Pakwan. Gettysburg College.
Q uality assessm ents ofch em oth erapy h ead-to-h ead trials A uth or C ontrolled F unding Y ear groupstandard Setting ofcare Type ofC h em o Pectasides Y es N R 2007 Single C enter Antiemetics Page 180 of 492 Final Report Update 1 Drug Effectiveness Review Project Evidence Table 3 discount 5mg haldol otc symptoms tuberculosis. C h em oth erapy:placebo-controlled trials A uth or Y ear A ge C ountry Study Design Interventions (drug R egim ent, G ender C h em o L evel Setting duration) Eligibility criteria Eth nicity A prepitant N avari M ulticenter A:D ay1:Apr400m g po Cisplatin-naïvepatients ≥18yearswho M ean:61. W om en Hesketh chem olevel5 B:D ay1:Apr400m g po of child-bearing agehadtohavea % M ale:62. E thnicity:N R C:D ays1-5:placebo PtsreceivedG ran+D ex 30m in beforecisplatinonD ay1 corticosteroids givenconcomitantly (see "A llowed oth ermedications") N C I:N ationalC ancerInstitute;U L N :U pperlimitofnormal Antiemetics Page 181 of 492 Final Report Update 1 Drug Effectiveness Review Project Evidence Table 3. C h em oth erapy:placebo-controlled trials A uth or N um ber N um ber Y ear screened/ with drawn/ C ountry eligible/ lostto A llowed oth erm edications/ C h em o L evel O th erpopulationch aracteristics enrolled fu/analyz ed interventions A prepitant N avari M eancisplatindose:79. C h em oth erapy:placebo-controlled trials A uth or Y ear C ountry M eth od ofadverse effects C h em o L evel R esults assessm ent A prepitant N avari A llcom parisons: G roupA vs. C 1999 A cute results (day 1): U SA N ovom iting:93% vs94% vs67% (p<0. C h em oth erapy:placebo-controlled trials A uth or Y ear Totalwith drawals; C ountry with drawals due to adverse C h em o L evel A dverse Effects R eported events A prepitant N avari C omparisons are made betweenG roups A vs B vs C ;and p=N S forall 1999 comparisons U SA (N umbers reported are % ofpts with th e A E) Hesketh chem olevel5 Clinicalevents: Constipation:19% vs13% vs18% D iarrhea:17% vs7% vs10% D ehydration:6% vs6% vs14% Headache:22% vs17% vs20% Hiccups:15% vs17% vs14% Asthenia:26% vs26% vs25% Hem atologic changes: D ecreaseintotalwhitecellcount:2% vs2% vs2% D ecreaseinneutrophils:0% vs2% vs2% Serum am inotransferaseelevations(transientincrease>2. C h em oth erapy:placebo-controlled trials A uth or Y ear C ountry C h em o L evel C om m ents A prepitant N avari 1999 U SA Hesketh chem olevel5 N C I:N ationalC ancerInstitute;U L N :U pperlimitofnormal Antiemetics Page 186 of 492 Final Report Update 1 Drug Effectiveness Review Project Evidence Table 3. C h em oth erapy:placebo-controlled trials A uth or Y ear A ge C ountry Study Design Interventions (drug R egim ent, G ender C h em o L evel Setting duration) Eligibility criteria Eth nicity C h awla M ulticenter A:D ay1:Apr40m g po Cisplatin-naïveptsage ≥18yrswhohad M ean:56. F em aleptsof childbearing potentialwere % W hite:58. D ays2-5:pts tookAprorplacebobetween8AM and10AM C orticosteroids givenconcomitantly; see "A llowed oth ermedications" N C I:N ationalC ancerInstitute;U L N :U pperlimitofnormal Antiemetics Page 187 of 492 Final Report Update 1 Drug Effectiveness Review Project Evidence Table 3. C h em oth erapy:placebo-controlled trials A uth or N um ber N um ber Y ear screened/ with drawn/ C ountry eligible/ lostto A llowed oth erm edications/ C h em o L evel O th erpopulationch aracteristics enrolled fu/analyz ed interventions C h awla M eancisplatindose:81. C h em oth erapy:placebo-controlled trials A uth or Y ear M eth od ofO utcom e C ountry A ssessm entand Tim ing of C h em o L evel DefinitionofO utcom es A ssessm ent C h awla Prim aryresponse:Com pleteresponse(C R ):noem etic episodesand Ptdiaryforem etic episodes 2002 norescuetherapyforD ays1-5 anduseof rescue International Hesketh chem olevel5 Totalcontrol(TC ):noem etic episodes,nouseof rescuetherapy,and 100m m N auseavisual m ax im um nauseaVAS<5m m analog scale(VAS): 0m m = nonausea Com pleteprotection(CP):noem esis,norescuetherapy,andno 100m m = nauseaasbadasit significantnausea(VAS<25m m ) couldbe N oem esis Ptsm arkedthisnauseaVAS everym orning (8AM -10AM ) N orescuetherapy forthenauseathey ex periencedthepreviousday. N onausea(m ax im um VAS <5m m ) Ptshadapost-studyvisit N osignificantnausea(m ax. VAS <25m m ) betweenD ay1and3days afterlastdoseof study Totalnum berof em etic episodes(0,1,2,≥3) m edication;andanothervisit betweendays19-29post cisplatinforF U andlab tests. N C I:N ationalC ancerInstitute;U L N :U pperlimitofnormal Antiemetics Page 189 of 492 Final Report Update 1 Drug Effectiveness Review Project Evidence Table 3. C h em oth erapy:placebo-controlled trials A uth or Y ear C ountry M eth od ofadverse effects C h em o L evel R esults assessm ent C h awla C omparisons are forgroups A (A pr40/25)vs. C (placebo) Tolerabilitywasm onitoredby 2002 A cute (Day 1): physicalex am s,including vital International CR :75. C h em oth erapy:placebo-controlled trials A uth or Y ear Totalwith drawals; C ountry with drawals due to adverse C h em o L evel A dverse Effects R eported events C h awla C omparisons:G roups A (40/25)vs B (125/80)vs C (placebo)vs D(375/250) 18/583= 3. C h em oth erapy:placebo-controlled trials A uth or Y ear C ountry C h em o L evel C om m ents C h awla TheApr375/250m g 2002 regim en(n= 34)was International replacedbytheApr40/25m g Hesketh chem olevel5 regim endueto pharm acokinetic dataand datashowing aninteraction betweenAprand dex am ethasone. N o statisticalcom parisonswere m adeforthisgroup,andthe resultsreportedwereforthe com pleteresponse: Acute:91%;D elayed:73%; O verall:70% N C I:N ationalC ancerInstitute;U L N :U pperlimitofnormal Antiemetics Page 192 of 492 Final Report Update 1 Drug Effectiveness Review Project Evidence Table 3. C h em oth erapy:placebo-controlled trials A uth or Y ear A ge C ountry Study Design Interventions (drug R egim ent, G ender C h em o L evel Setting duration) Eligibility criteria Eth nicity de W it M ulticenter A:D ay1:Apr375m g Cisplatinnaïvepatients ≥ 18years,who M ean:57. C h em oth erapy:placebo-controlled trials A uth or N um ber N um ber Y ear screened/ with drawn/ C ountry eligible/ lostto A llowed oth erm edications/ C h em o L evel O th erpopulationch aracteristics enrolled fu/analyz ed interventions de W it M eancisplatindose:80. C h em oth erapy:placebo-controlled trials A uth or Y ear M eth od ofO utcom e C ountry A ssessm entand Tim ing of C h em o L evel DefinitionofO utcom es A ssessm ent de W it Com pleteresponse:noem esisandnorescuetherapy 2003 International Partialresponse:0-2em etic episodesandnorescuetherapy Hesketh chem olevel5 F ailedresponse:>2em etic episodesand/oruseof rescuetherapy (thisstudypopulationseem s tobethepre-dose adjustm entcadrefrom the Chawlapaper) Thisstudylookedat6cycles of chem o;dataforCycles1 & 2 onlyareabstractedhere N C I:N ationalC ancerInstitute;U L N :U pperlimitofnormal Antiemetics Page 195 of 492 Final Report Update 1 Drug Effectiveness Review Project Evidence Table 3. C h em oth erapy:placebo-controlled trials A uth or Y ear C ountry M eth od ofadverse effects C h em o L evel R esults assessm ent de W it Cycle1data:(G roup B(n= 80)vs. C(n= 38)) tobethepre-dose % Com pleteresponse:80% vs71%,p= N R adjustm entcadrefrom the % Partialresponse:10. C h em oth erapy:placebo-controlled trials A uth or Y ear Totalwith drawals; C ountry with drawals due to adverse C h em o L evel A dverse Effects R eported events de W it C omparisons:G roups A (375/250,n=23)vs B (125/80,n=62)vs C (placebo, 2003 n=60) International F orA Es incycles 2-6 Hesketh chem olevel5 % with ≥ 1adverseevent(AE s):74vs76vs73 % with drug-relatedAE s:26vs34vs25 (thisstudypopulationseem s % with seriousAE s:9vs26vs15 tobethepre-dose % discontinuedduetoAE s:13vs10vs10 adjustm entcadrefrom the % with ≥1laboratoryAE :22vs26vs27 Chawlapaper) % with drug-relatedlaboratoryAE :0vs7vs5 W ith m ostcom m onAE s(≥10% inatleast1treatm entgroup): Thisstudylookedat6cycles Abdom inalpain:9vs10vs10 of chem o;dataforCycles1 F atigue:26vs18vs17 & 2 onlyareabstractedhere D ehydration:0vs13vs10 D iz z iness:9vs13vs10 Influenz a-likedisease:13vs2vs2 Constipation:22vs10vs13 D iarrhea:9vs23vs13 D ysgeusia:17vs5vs7 N ausea:17vs18vs13 Anem ia: 13vs7vs13 F ebrileneutropenia:0vs11vs2 Headache:4vs11vs15 Hiccups:9vs15vs8 D yspnea:13vs2vs5 N C I:N ationalC ancerInstitute;U L N :U pperlimitofnormal Antiemetics Page 197 of 492 Final Report Update 1 Drug Effectiveness Review Project Evidence Table 3.
Because these studies included unselected populations discount haldol 5 mg otc treatment quotes, findings were probably more applicable to the average rheumatoid arthritis patient than results from efficacy trials. Limitations with respect to risk of bias have to be kept in mind though. Sponsorship All trials were funded by the pharmaceutical industry. Meta-analyses and cohort studies usually had public or a mix of public and industry funding. Detailed assessment: Direct evidence on comparative effectiveness Overall, we included eight head-to-head studies comparing one targeted immune modulator to 39-45,48 another. These direct comparisons, however, were limited to abatacept compared with infliximab, adalimumab and etanercept compared with infliximab, and adalimumab compared Targeted immune modulators 29 of 195 Final Update 3 Report Drug Effectiveness Review Project with etanercept. We could not find any head-to-head evidence for any of the other drugs. Abatacept compared with infliximab The only double-blinded head-to-head trial, the ATTEST (Abatacept or infliximab compared with placebo, a Trial for Tolerability, Efficacy, and Safety in Treating rheumatoid arthritis) 39 study, was a fair randomized controlled trial that compared abatacept with infliximab. This study enrolled 431 patients and randomized them to abatacept (10 mg/kg every 4 weeks + methotrexate), infliximab (3 mg/kg every 8 weeks + methotrexate), or placebo. The primary outcome was assessed at 6 months followed by a double-blinded extension phase up to 1 year. No statistically significant differences in efficacy were obvious between treatments at 6 months (DAS 28: abatacept ‒2. At 1 year, however, significantly more patients on abatacept than on infliximab achieved American College of Rheumatology 20 response (American College of Rheumatology 20 response 72. Likewise, health-related quality of life measures (Health Assessment Questionnaire Disability Index, Short Form 36 Health Survey) improved statistically significantly more with abatacept than with infliximab treatment. It has to be noted though, that infliximab was administered at a fixed dose regimen throughout the entire study. Infliximab efficacy trials have shown that up to 30% of patients require dose increases. Adalimumab compared with etanercept The evidence on the comparative effectiveness of adalimumab and etanercept is limited to a 44 45 good and a fair observational study. Both studies were based on national registers of targeted immune modulators (the Danish DANBIO [Danish Biological] and the Dutch DREAM [Dutch Rheumatoid Arthritis Monitoring]) and were conducted prospectively in primary care based populations. Both studies enrolled patients who had failed at least one conventional disease- modifying antirheumatic drug and were started on a targeted immune modulator. The choice of the treatment and dosing was at the discretion of the treating rheumatologist. Overall, 356 44 patients were followed up for 12 months in the study based on the DREAM register, and 969 45 patients in the study based on the DANBIO register. After 12 months of follow-up, treatment responses in both studies were similar for patients on adalimumab and etanercept. The primary outcome of the DREAM study was the 44 DAS28 course over a 12 months follow-up, as analyzed on an intent-to-treat basis. At study endpoint patients on adalimumab and etanercept had similar improvements of the DAS28 (‒1. Results of the DANBIO study were not based on an intent-to-treat principle (patients who withdrew from treatment before 6 months were excluded). Results, however, also presented similar effectiveness between adalimumab and etanercept. The LUNDEX corrected ([fraction of starters still in the study after given months] x [fraction responding at given months]) American College of Rheumatology 50 response was 35% for adalimumab and 32% 45 for etanercept after 12 months. Discontinuation rates in both studies were similar in patients on Targeted immune modulators 30 of 195 Final Update 3 Report Drug Effectiveness Review Project adalimumab and etanercept (e. Adalimumab compared with infliximab The same prospective cohort studies based on the DREAM and the DANBIO registers described 44,45 above also compared the effectiveness of adalimumab with infliximab. In both studies, patients treated with adalimumab had statistically significantly better response rates after 12 months of follow-up than patients treated with infliximab. For example, in the DREAM study (N=418), patients on adalimumab had statistically significantly greater improvements on the DAS28 (‒1. Likewise, in the Danish DANBIO study (n=1452), 35% of patients treated with adalimumab achieved a LUNDEX-corrected American College of Rheumatology 50 response at 12 months, compared with 25% of patients 45 on infliximab (P<0.
Funding: The Drug Effectiveness Review Project purchase haldol 10 mg amex medicine 5000 increase, made up of 15 organizations including 14 state Medicaid agencies, commissioned and funded this report. These organizations selected the topic of the report and had input into its Key Questions. Content and conclusions of the report were determined entirely by researchers at the Evidence-based Practice Center. The authors of this report have no financial interest in any company that makes or distributes the products reviewed in the report. Overactive bladder Page 4 of 73 Final Report Update 4 Drug Effectiveness Review Project INTRODUCTION Overactive bladder is defined by the International Continence Society as a syndrome of urinary frequency and urgency, with or without urge incontinence, appearing in the absence of local 1 1 pathological factors. Urinary continence relies heavily upon control and coordination of the smooth muscle found in the wall of the bladder. The effective storage of urine relies on detrusor muscle relaxation, and contraction of internal and external sphincters found within the neck of the bladder while voiding is controlled through the contraction of the bladder’s detrusor muscle and relaxation of its internal and external 2 sphincters. Bladder contraction is mediated via cholinergic muscarinic receptors in bladder smooth muscle. The most common cause of overactive bladder syndrome is detrusor overactivity. Detrusor overactivity may be either idiopathic or neurogenic in origin. A subset of patients with an overactive bladder may complain of urge urinary incontinence, involuntary 3, 4 leakage accompanied by or immediately preceded by urgency. Overactive bladder has been estimated to affect 20% of community-dwelling senior citizens and 2, 5 around 50% of institutionalized elderly persons. Independent risk factors for the development of overactive bladder include neurologic impairment, immobility, female gender, and history of hysterectomy. It is common for urge incontinence to coexist with stress incontinence, especially in women. Treatment of overactive bladder syndrome first requires a clear diagnosis. In patients with incontinence, multiple forms can be present and it is important to determine which form is dominant. Non-pharmacologic, non-surgical treatment consists of behavioral training (prompted voiding, bladder training, pelvic muscle rehabilitation), transcutaneous electrical nerve 6 stimulation, catheterization, and use of absorbent pads. Pharmacologic treatment for overactive bladder syndrome includes darifenacin, flavoxate hydrochloride, hyoscyamine, oxybutynin chloride, tolterodine tartrate, trospium chloride, scopolamine transdermal, and solifenacin succinate. Flavoxate hydrochloride acts as a direct spasmolytic on smooth muscle and maintains 2, 7 anticholinergic as well as local analgesic properties. Oxybutynin chloride has direct antispasmodic action on smooth muscle and inhibits the muscarinic action of acetylcholine on 2, 7, 8 2, 7, 9 smooth muscle. Tolterodine tartrate acts as a competitive muscarinic receptor antagonist. Anticholinergic agents have been included in a number of expert-opinion based reviews of drugs with high risk of adverse effects in the elderly. These papers include oxybutynin as an example of an anticholinergic drug with this potential, but evidence linking oxybutynin to adverse events is not presented. Because these reviews are not systematic, and do not make comparisons to any of the other drugs included in this report, we do not include these papers here. The purpose of this systematic review is to compare the benefits and harms of drugs used to treat overactive bladder syndrome. Overactive bladder Page 5 of 73 Final Report Update 4 Drug Effectiveness Review Project Purpose and Limitations of Systematic Reviews Systematic reviews, also called evidence reviews, are the foundation of evidence-based practice. A systematic review focuses on the strength and limits of evidence from studies about the effectiveness of a clinical intervention. Systematic reviews begin with a careful formulation of research questions. The goal is to select questions that are important to patients and clinicians, then to examine how well the scientific literature answers those questions. Terms commonly used in systematic reviews, such as statistical terms, are provided in Appendix A and are defined as they apply to reports produced by the Drug Effectiveness Review Project. Systematic reviews emphasize the patient’s perspective in the choice of outcome measures used to answer research questions. Studies that measure health outcomes (events or conditions that the patient can feel, such as fractures, functional status, and quality of life) are emphasized over studies of intermediate outcomes (such as change in bone density).
The field is eagerly controlled trial in healthy volunteers showed no induction of awaiting the outcome of the ongoing trials haldol 10mg medications xerostomia. However, due to the standard practice of giving data raise the possibility that human biology simply differs from fresher blood to neonates, the mean age of RBCs in the “fresh” and murine and canine biology in this regard. Therefore, it consistent with the data are the hypotheses that multiple units must is unclear whether ARIPI had a group with old enough RBCs to test be given and that the effect would be augmented in sick patients the hypothesis in a broader context. However, what is clear is that in with baseline innate immune activation. Recent studies lend some 652 American Society of Hematology support to this latter notion in patients experiencing trauma. Therefore, the very general hypothesis that “older blood results in after transfusion in neonates, although fresh RBCs were not worse medical outcomes” covers the essence of what one would compared with older RBCs in that study. However, the more generalized a hypothesis, the more one into a pediatric population, with less induction from washed units. The result can lead to questions that are essentially meaningless in substance and impos- In addition to inducing cytokines in mice and dogs, transfusion of sible to answer. Although it is not clear that this is the case with older units of RBCs resulted in plasma factors that support the testing the hypothesis that “older blood results in worse medical growth of ferrophilic bacteria in either mice or human speci- outcomes,” it is necessary to give careful attention to this issue and mens. Solomon et al recently reported a study on dogs receiving a massive exchange transfusion after a pulmonary inoculation with Staphylo- One source of generalization in the question of whether “older blood coccus aureus. Blood transfusions are given for a wide infection, with increased tissue necrosis. These findings coincided number of different indications. The physiological effects of with findings indicating hemolysis in vivo, such as increased NO exposure to the biochemical changes that occur in a stored blood consumption and decreased haptoglobin. The above findings and product may be vastly different depending upon the pathophysiol- reports are generating a body of evidence implicating older RBCs in ogy of the transfusion recipient. Some retrospective trials have systemic inflammation and the potential promotion of bacterial looked at all hospitalized patients who were transfused within a infection in nonhuman animal models. However, other retrospective trials are It has been reported that stored RBCs acquire procoagulant activi- 30-33 certainly more focused and prospective trials are inevitably so due ties. These activities include changes in Russell viper venom 30 32 31 to the need to limit sample size as a practical matter. Nevertheless, time, clotting activity, increased thrombin generation, and 33 even in the narrower context, the problem persists. Although the mechanisms of these activities are not entirely elucidated, there are data to implicate Consider a trial that is focused on patients in a particular category of microparticles, the exposure of phospholipids, and a potential role disease (eg, trauma patients arriving at the emergency department, for tissue factor. Although provocative, the results of these in vitro patients with sickle cell disease, patients admitted to the intensive studies have yet to be transitioned into in vivo observations. Even within these definitions, which are clearly narrower than generalized Advanced glycation end products populations, there is a distinct heterogeneity of recipient pathology One of the effects upon exposure of proteins to glucose is a reaction that may alter the effects of stored blood transfusion. For example, between the aldehyde group of glucose with free amino groups, consider patients admitted to the intensive care unit. For the sake of leading to a Schiff base that rearranges into a series of advanced example, let us also assume that free hemoglobin in stored RBCs is glycation end (AGE) products, including carboxy-methyl-lysine. Overall, AGEs constitute a complex class of care patients may be suffering from insufficient blood flow to a vital molecular glycation with diverse structures. There are several receptors that have been described with the capacity to recognize organ (eg, thrombotic disease, atherosclerotic stenosis, etc). Most notably is the receptor for a patient, it is reasonable to predict that impairing vascular advanced glycation end products (RAGE). RAGE plays an active relaxation would exacerbate their condition and may lead to a worse role in inflammation and innate immune activation. However, another subset of intensive care patients may are stored in supraphysiological levels of glucose, it has been have a pathophysiology in which insufficient vessel tone is playing a hypothesized that AGEs would be increased as a result of storage. In this case, it is possible that the scavenging of (ie, carboxy-methyl-lysine) and that they are capable of ligating NO may do no harm and may even have a therapeutic effect, RAGE, leading to alterations of cultured endothelial cells. Similarly, procoagu- play a role in inflammatory pathologies posttransfusion, such as lant activity in stored RBCs may be harmful to a patient with transfusion-related acute lung injury. Indeed, biologies of this nature could Testing the central hypothesis go a long way toward explaining why, in some settings, fresher It seems clear, or at least very likely, that retrospective approaches blood seems to be worse than older blood. The result of combined analyses of retrospective findings leads to a murky view with an In the above scenario, it is easy to see that even very real effects on equivocal outcome.