Zestoretic
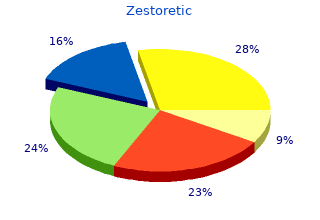
2018, Wheaton College, Massachusetts, Iomar's review: "Zestoretic generic (Lisinopril-hctz) 17.5 mg. Effective Zestoretic.".
Overall low energy/activity level can also be a possible indicator of a thyroid problem buy 17.5 mg zestoretic amex arrhythmia 101, but could be caused by other factors also. However, some laboratory reference ranges may be too broad, and should be interpreted carefully. Treatment: If iodine levels are low, then one can begin with iodine supplementation. If that does not normalize thyroid levels, then one can consider thyroid supplements. We recommend natural thyroid supplements derived from animals, as they will provide a complete thyroid source. Duration: Usually 1-2 months of supplementation is needed to observe an increase in energy level and body temperature. Supplementation may be needed long-term unless the problem with thyroid development is resolved. Research: One study by Adams et al found that many children with autism have unusually low levels of iodine in their hair, which possibly suggests a low level in their body and need for more. Analyses of toxic metals and essential minerals in the hair of Arizona children with autism and associated conditions, and their mothers. Some children with autism have a low level of sulfate in their bodies, due to a variety of reasons including poor absorption in the gut, excess loss in the urine, poor recycling of sulfate by the kidney, or oxidant stress and inflammation can shut down cysteine dioxygenase, which throttles the cysteine -> sulfate route. Testing: Blood testing can be used to check for levels of free and total plasma sulfate, and this is probably the more reliable test. Alternatively, since Epsom salt baths are very safe, one could simply try them for up to several weeks and look for improvements in behavior and functioning (see below). Treatment: Tapan Audhya evaluated many different ways to increase plasma sulfate levels in children with autism who had low levels. Research: A small pilot study by Alberti et al found that children with autism had a reduced sulfation capacity compared to controls. They include: 1) Oral glutathione: Only about 10% of oral glutathione is absorbed, so this method is not very effective at raising body levels, but it may improve levels in the gut. Adding subcutaneous injections of methyl-Vitamin B12 (methyl-cobalamin) resulted in normalization of plasma glutathione levels. Research: A large study by James et al confirmed her original finding of low glutathione in children with autism due to abnormalities in their methionine pathway. She also found that children with autism were more likely to have genetic polytypes associated with abnormalities in the methionine pathway. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Glutathione is the body’s primary defense against mercury, toxic metals, and many toxic chemicals, so a low level of glutathione results in a higher body burden of toxins. Also, many children with autism had increased use of oral antibiotics in infancy, which alter gut flora and thereby almost completely stop the body’s ability to excrete mercury. Normalizing glutathione, restoring gut flora, and removing toxic metals often results in reduction of the symptoms of autism. Preparation for Treatment: Prior to beginning chelation, it is important to first prepare the body for it. This includes: 1) Reducing exposure to toxins (organic food, reverse osmosis water, no mercury fillings, avoiding pesticides, etc. They include: 1) Urinary porphyrins: This test checks for abnormal levels of porphyrins in the urine, where different porphyrin levels appear to correlate with body burden of mercury, lead, or other toxic metals. A large increase indicates that the metals are present, and that the medication is helpful in removing them. Children may have a high body burden but a low level in their current hair, blood, or urine. Some of the compounded rectal suppositories also appear to increase excretion of toxic metals, but the transdermal forms do not measurably increase excretion of toxic metals. Consensus Report on Treating Mercury Toxicity in Children with Autism, available at www. This report provides much more detailed advice on pre-treatments, treatments, dosages, and safety. The data includes: 1) A literature review by Bernard et al showing that the symptoms of autism were very similar to those of people suffering from infantile exposure to mercury poisoning. James et al, Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism.
Second-generation antipsychotic-associated diabetes mellitus and diabetic ketoacidosis: mechanisms discount zestoretic 17.5mg on-line arrhythmia facebook, predictors, and screening need [American Society of Clinical Psychopharmacology Corner]. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. Risperidone in children and adolescents with pervasive developmental disorder: pilot trial and follow-up. Prolactin levels during long-term risperidone treatment in children and adolescents. Prolactin levels in young children with pervasive developmental disorders during risperidone treatment. A prospective study of hyperprolactinemia in children and adolesceents treated with atypical antipsychotic agents. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Antipsychotic-induced hyperprolactinemia: mechanisms, clinical features and management. Quetiapine: are we overreacting in our concern about cataracts (the beagle effect)? Practice parameter on the use of psychotropic medications in children and adolescents. Aripiprazole in Children and Adolescents with Tourette‟s Disorder: An Open-Label Safety and Tolerability Study. A double-blind placebo-controlled trial of sibutramine for olanzapine associated weight gain. Bipolar Disorder Advocacy 51 Author and Expert Consultant Disclosures and Contributing Organizations 52 References 55 The information contained in this guide is not intended as, and is not a substitute for, professional medical ParentsMedGuide. Two decades ago, it was rare for a child or adolescent to be diagnosed with bipolar disorder. Research now suggests that for some, the symptoms of adult bipolar disorder can begin in childhood. However, it is not yet clear how many children and adolescents diagnosed with bipolar disorder will continue to have the disorder as adults. What is very clear is that obtaining a careful clinical assessment is utmost and critical to diagnosing bipolar disorder. During the past decade, the number of children and adolescents diagnosed with bipolar bipolar disorder has increased signifcantly. Yet we do not understand why bipolar disorder is being diagnosed more frequently in children. We suspect that it is because of an increased awareness of the disorder as well as over diagnosis. However, we all agree that children who have issues with mood and behavior need help. Recent research and clinical experience has provided child and adolescent psychiatrists with a better understanding of bipolar disorder and its symptoms. There are still many unanswered scientifc questions about how to best diagnose and treat bipolar disorder in children and adolescents. However, the body of research evidence and clinical consensus on this disorder is growing. The information con- tained in this medication guide refects what medications child psychiatrists currently use when treating bipolar disorder during childhood and adolescence. The guide is intended to provide parents with the latest expert medical opinion about medications used to treat the symptoms of bipolar disorder. While research is ongoing to better understand the benefts and risks of using these medications, only a limited number of these drugs have been approved by the U. For more information about the Parents Medication Guide series of publica- tions, please visit http://www.
A trial of the effcacy buy zestoretic 17.5 mg online hypertension 6 weeks postpartum, safety and impact on drug resistance of four drug regimens for seasonal intermittent preventive treatment for malaria in Senegalese children. The effect of dosing strategies in the therapeutic effcacy of artesunate-amodiaquine for uncomplicated malaria; a meta- analysis of individual patient data. Use of weight-for-age-data to optimize tablet strength and dosing regimens for a new fxed-dose artesunate–amodiaquine combination for treating falciparum malaria. Structure and mechanism of action F H3C H3C Artemether is the methyl ether derivative ofH3C F F F dihydroartemisinin. It is two- to threefold less F N O O active than dihydroartemisinin, its active O O C O C O C F O O metabolite. The pharmacokinetics of oral artemether when given in the fxed-dose combination with lumefantrine for the treatment of uncomplicated malaria is shown in section A5. Artemether is a water-insoluble, lipid-soluble compound and is therefore given either as an oil-based intramuscular injection or orally. It is absorbed slowly and erratically after intramuscular administration in severe malaria (Figure A5. While dihydroartemisinin is responsible for most of the antimalarial action after oral administration, the concentrations of artemether parent compound predominate after intramuscular administration in severe A falciparum malaria. Artemether also undergoes auto-induction but to a lesser 5 extent than artemisinin. Both artemether and dihydroartemisinin are eliminated within 7 h of administration (3, 5–10). Individual concentration–time profles for artemether after the frst intramuscular dose of 3. Safety Adverse effects Artemether is generally very well tolerated after both oral and intramuscular administration. It has similar side-effects to other artemisinin derivatives, including hypersensitivity reactions (risk estimate, 1 in 3000), mild gastrointestinal disturbance, dizziness, reticulocytopenia, neutropenia and elevated liver enzyme activity. While studies in experimental animals show neurotoxicity after parenteral artemether, clinical, neurophysiological and pathological studies in humans have not shown similar fndings. Contraindications Artemether is contraindicated in patients with known hypersensitivity to any artemisinin derivative. Cautions A marked increase in the concentration of artemether in the cerebrospinal fuid of patients with meningitis was observed, prompting researchers to advise caution in treating patients with signs of meningitis (2, 10, 11). Patients with acute renal failure have higher maximum concentrations, higher exposure, a lower volume of distribution and a longer elimination half-life of artemether than people without renal failure (6). Comparative pharmacokinetics of intramuscular artesunate and artemether in patients with severe falciparum malaria. The disposition of intramuscular artemether in children with cerebral malaria; a preliminary study. Pharmacokinetics of intramuscular artemether in patients with severe falciparum malaria with or without acute renal failure. Artesunate suppositories versus intramuscular artemether for treatment of severe malaria in children in Papua New Guinea. Population pharmacokinetics of artemether and dihydroartemisinin following single intramuscular dosing of artemether in African children with A severe falciparum malaria. Artemether bioavailability after oral or intramuscular administration in uncomplicated falciparum malaria. Meningeal infammation increases artemether concentrations in cerebrospinal fuid in Papua New Guinean children treated with intramuscular artemether. Pharmacokinetic parameters estimated for artemether, lumefantrine and their respective active metabolites, dihydroartemisinin and desbutyllumefantrine in studies of currently recommended doses of artemether– lumefantrine used for treatment of acute malaria (range of mean or median values reported). Parameter Artemether Dihydroartemisinin Lumefantrine Desbutyl- lumefantrine Cmax (ng/mL) 5. Lumefantrine is highly lipophilic and is more readily absorbed when co-administered A 5 with fatty foods or milk (4, 5, 7). Its bioavailability and the time to reach maximum concentrations vary within and between individuals, primarily due to fat-dependent absorption.
Long term results were often not available and complications varied by study (frequently they were not reported) in the literature available for this guideline buy zestoretic 17.5mg otc heart attack is recognized by a severe pain. Most treatments are associated with some known risks, especially invasive and operative treatments. We developed systematic reviews for this guideline because these reviews employ specific processes designed to minimize bias in the 6, 7 selection, summary, and analysis of this literature. In referring to bias, we explicitly mean both the biases that can arise from financial conflicts of interest and biases that can arise from intellectual conflicts if interest. This section of the present document describes how we conducted our systematic reviews and how the guideline was developed. Accordingly, in this section we describe our strategies for finding relevant literature, our criteria for selecting articles to include in this guideline, how we extracted data, how we appraised and graded the evidence, our methods of statistical analysis, and the review and approval steps this guideline went through. Elsewhere in this document, we provide extensive documentation so that interested readers can assure themselves that we attempted to combat bias wherever possible. The work group met again on July 31 and August 1, 2009 to write and vote on the final recommendations and rationales for each recommendation. These recommendations specify [what] should be done in [whom], [when], [where], and [how often or how long]. They function as questions for the systematic review that underpins each preliminary recommendation, and they do not function as final recommendations or conclusions. Once established, these a priori preliminary recommendations cannot be modified until the final work group meeting. The a priori and inviolate nature of the preliminary recommendations combats bias by preventing a “change in course” if a systematic review yields results that are not to someone’s liking. The results of each systematic review are presented and discussed at the final work group meeting. At this time the preliminary recommendations are modified in response to the evidence in the systematic review. These criteria are our “rules of evidence” and articles that do not meet them are, for the purposes of this guideline, not evidence. To be included in our systematic reviews (and hence, in this guideline) an article had to be a report of a study that: • Evaluated a treatment for acute Achilles tendon rupture. Acute Achilles tendon ruptures are defined as a rupture treated within zero to six weeks post injury. We included surrogate outcomes only when patient-oriented outcomes were not available. Surrogate outcomes are laboratory or other measurements that are used as 9 substitutes for how a patient feels, functions, or survives. We only included data for an outcome if ≥ 50% of the patients were followed for that outcome. For example, some studies report short-term outcomes data on nearly all enrolled patients, and report longer-term data on less than half of the enrolled patients. Additionally, we downgraded the Level of Evidence by one in instances where 50% to ≤80% of patients were followed. We only included data for outcomes reporting the average length of time to return to an activity if >80 % of the patients were included in the calculation. For example, some studies report the mean time for return to work as 6 weeks but are only including data for patients who have actually returned to work and are ignoring patients who are unable to return. Using comprehensive literature searches ensures that the evidence we considered for this guideline is not biased for (or against) any particular point of view. Strategies for searching electronic databases were constructed by a Medical Librarian and reviewed by the work group. All searches of electronic databases were supplemented with manual screening of bibliographies of all retrieved publications. We also searched the bibliographies of recent systematic reviews and other review articles for potentially relevant citations.